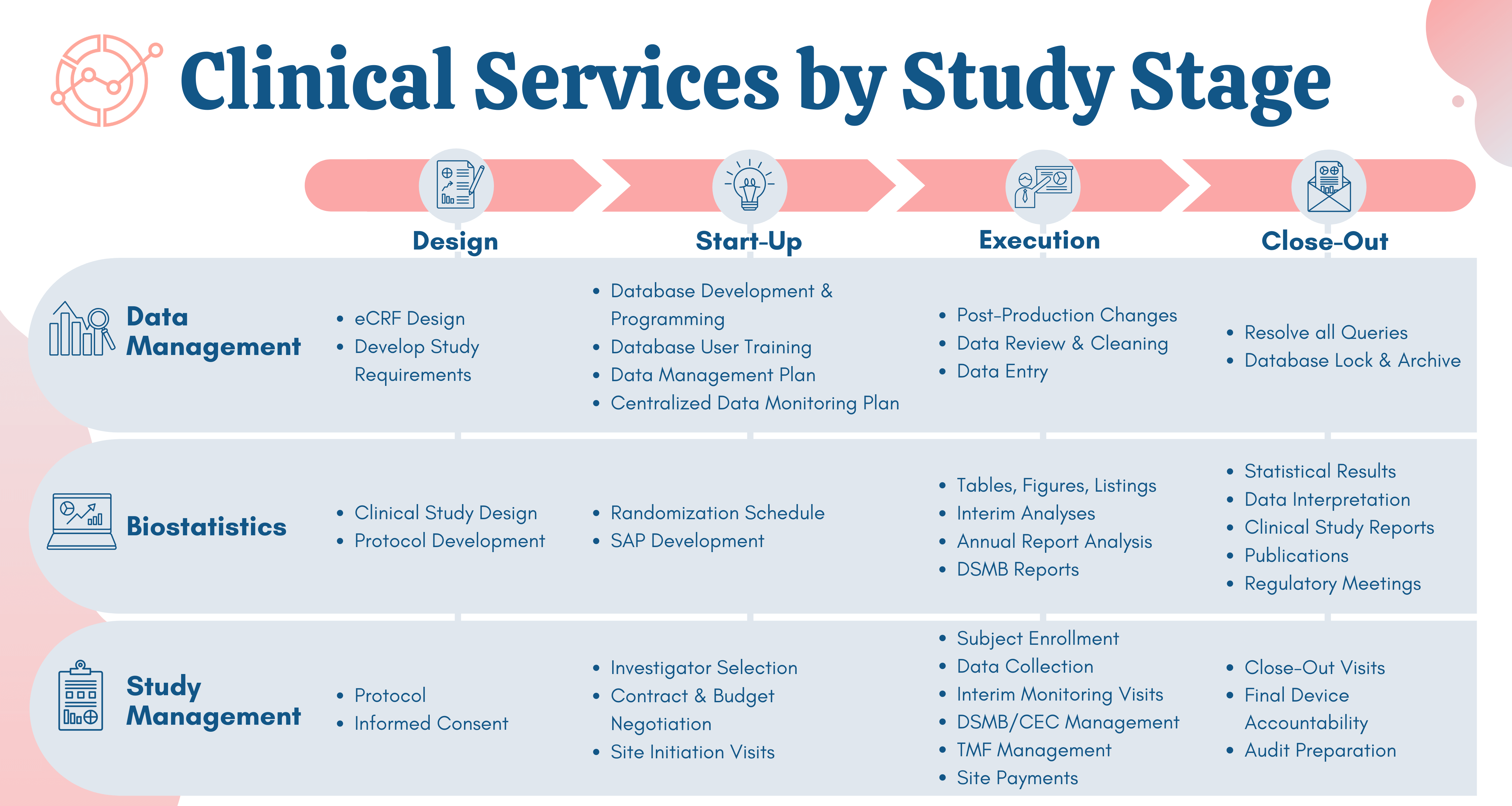
The global medical device landscape is complex. Getting a new product or therapy approved requires compelling clinical evidence. At NAMSA, we know what you’re up against and understand how to set you up for success, which is why we offer expert medical device clinical research consulting services.
Our clinical trial consulting expertise spans every manner of technology, therapy, indication and geography. This broad range of experience allows us to successfully lead our clients through all phases of clinical research: from first-in-human to pivotal and post-market. NAMSA’s global footprint also provides clients direct access to local networks to conduct safe, effective and efficient clinical trials, which are optimized to achieve regulatory approval and continued innovation.